Asthma

Asthma
Definition
Asthma is a chronic inflammatory lung disease characterized by airway inflammation, narrowing & bronchial hyperreactivity, leading to episodic symptoms.
Phenotypes
Asthma can be classified into clinical and inflammatory subtypes, where the latter being more specific in guiding treatments.
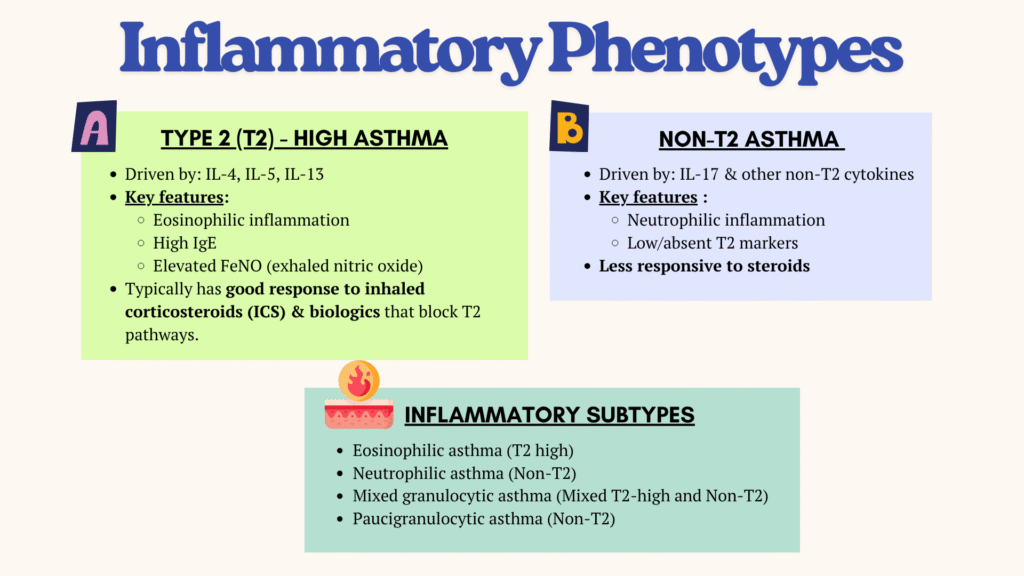
In terms of clinical phenotypes, some common ones include:
- Allergic asthma (eosinophilic inflammation)
- Non-allergic asthma (may be neutrophilic, eosinophilic or paucigranulocytic)
- Cough variant asthma & cough predominant asthma
- Adult-onset (late-onset) asthma
– Tend to be non-allergic, often requiring higher doses of ICS, or are relatively refractory to corticosteroid treatment.
– Have to rule out occupational asthma - Asthma with persistent airflow limitation – due to airway remodelling
- Asthma with obesity – different pattern of airway inflammation, with little eosinophilic inflammation
Diagnosis
The diagnosis of asthma requires combination of clinical history + physical examination + lung function test (evidence of airway obstruction variability)
Clinical history
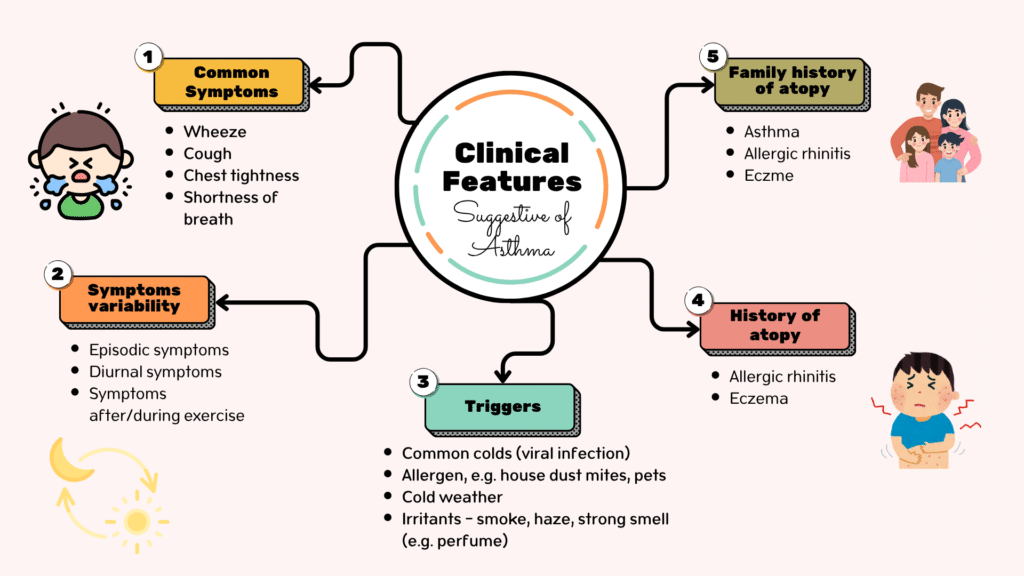
Physical examination
- Audible wheeze
- Rhonchi on auscultation
- Use of accessory muscles
Lung function test
Spirometry is the recommended tool to demonstrate airway obstruction/variability/reversibility.
However, if spirometry is not available, peak flow meter may be used instead (better than no objective measurement).
Ideally, lung function test should be done before starting treatment.
A low FEV1 indicates underlying untreated airway inflammation & is a risk factor for future exacerbation.
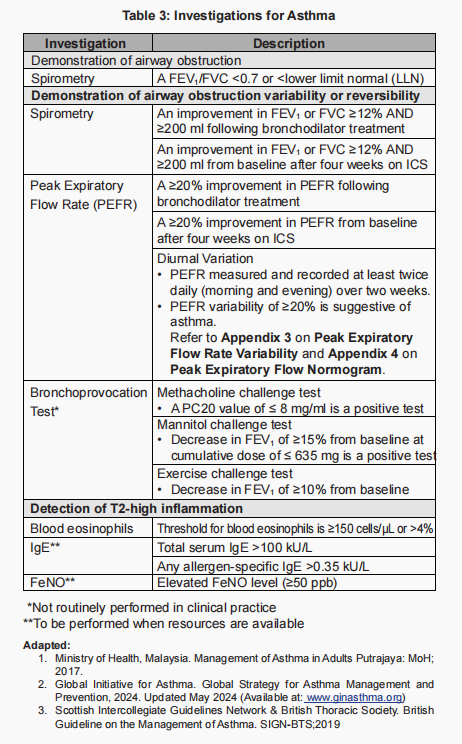
If spirometry or PEF is unavailable, and variable airflow limitation cannot be confirmed:
- Decision to start treatment depends on clinical urgency & access to further testing.
- In urgent situations, it may be reasonable to start ICS-containing treatment empirically while arranging for further evaluation.
Role of biomarkers
Our latest CPG and the GINA 2025 guidelines highlight the role of biomarkers—particularly blood eosinophils and FeNO (fractional exhaled nitric oxide)—in asthma assessment. These tests help determine the patient’s asthma phenotype and guide decisions on starting biologic therapy. However, they are often not readily available in primary care settings.
In addition, FeNO can be used to monitor treatment response. Persistently elevated FeNO after starting ICS may suggest poor adherence or inadequate ICS dosage.
Confirming diagnosis in patients who are already on treatment (bronchodilator/ICS)
1. Assessing treatment response
- Improvement in treatment –> supports diagnosis of asthma
- Lack of response –> does not rule out asthma (may still need objective testing)
2. Consider spirometry
- Perform spirometry ideally after withholding bronchodilators
– SABA: > 4 hours
– LABA: 24 – 48 hours
– GINA recommends: > 4h (SABA); > 24h (BD ICS-LABA); > 36h (OD ICS-LABA_ - Alternatively, spirometry can be performed when symptomatic.
Asthma Control
After confirming the diagnosis of asthma, the next step is to assess for asthma control.
Various tools are available to assess patient’s asthma control.
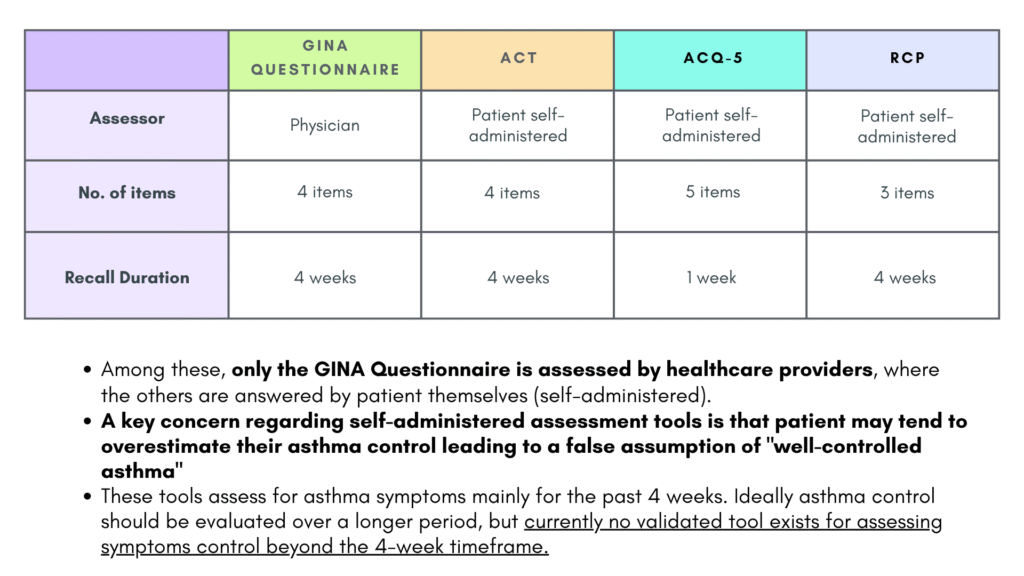
GINA assessment tool
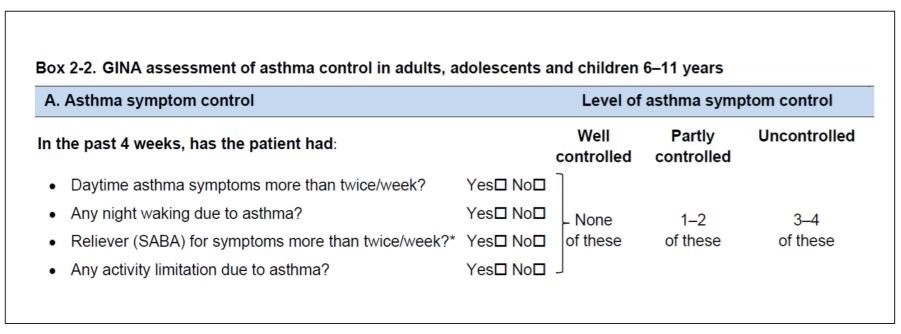
Do not include reliever taken before exercise, as some people take this routinely without knowing whether they need it.
Note that the reliever mentioned here is only applicable for SABA and not anti-inflammatory reliever like ICS-formoterol
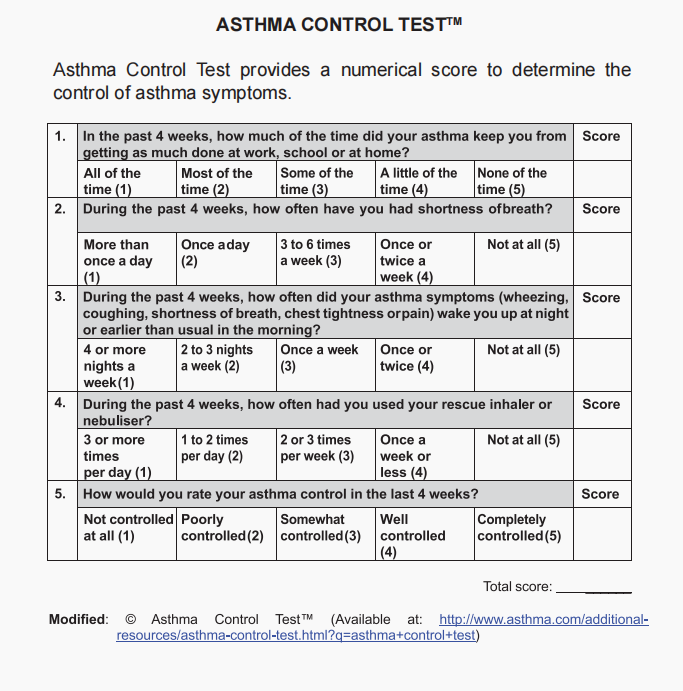
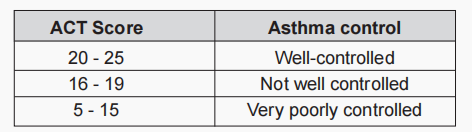
Asthma Control Test
GINA recommendation
- Avoid using the term “mild asthma” – it can mislead patients/clinicians into thinking there is low risk and no need for ICS.
- Even patients with infrequent/mild symptoms can have severe or fatal exacerbations if treated with SABA alone.
- Always emphasize the need for ICS-containing therapy for all asthma patients, regardless of symptom frequency, severity, or risk factors.
Asthma Treatment Goals 🎯
1. Optimal symptom control – few/no asthma symptoms, no sleep disturbance and unimpaired physical activity
2. Risk reduction in terms of :
- Asthma-related mortality
- Exacerbations
- Persistent airflow limitation
- Side-effects of treatment
- Minimize risk of future exacerbations
- Reducing treatment side effects
- Preventing persistent airflow limitation
- Lowering asthma related mortality
Non-pharmacological treatment
Complements pharmacological therapy to improve control and quality of life.
These include:
1. Lifestyle modifications
- Stop smoking/vaping, including avoidance of passive smoking
- Physical activity
– Improved cardiopulmonary fitness & subsequently better asthma control.
– Although physical activity does not generally confer specific benefit on lung function, swimming in young people has shown improved lung function.
– Caution: Exercise-induced bronchoconstriction (EIB)* - Breathing exercise & yoga
– May improve symptoms & QoL
– Helps manage emotional stress (trigger for asthma)
– Limited effect on lung function or exacerbations
2. Pulmonary rehabilitation – structured programs can improve exercise capacity & QoL
3. Diet & weight management
- Diet – GINA guideline advocates diet rich in fruit & vegetables for its general health benefits, which may include lower risk of asthma & lung function decline.
- Weight management – especially for obese & overweight individuals
– Weight reduction program plus twice-weekly aerobic and strength exercises is more effective for symptom control than weight reduction alone.
– Bariatric surgery gives greatest improvement in asthma outcomes.
4. Allergen & Environmental control
- Avoid outdoor activities during poor air quality, cold weather, or low humidity.
- Removing pets from home if allergic to animal dander
- Reduce household dampness to prevent mold growth.
5. Vaccination, which may include:
- Influenza
- Pneumococcal (recommended especially for those on maintenance corticosteroids or frequently repeated systemic corticosteroids)
- RSV, Covid-19 (GINA guideline)
6. Medication precautions
- Aspirin & NSAIDs
– Generally not contraindicated unless there is h/o adverse reactions.
– If needed, COX-2 inhibitors are preferred (e.g. celecoxib or etoricoxib) - B-blockers (including eye drops)
– Avoid non-selective B-blockers.
– Cardio-selective B-blockers may be used with caution.
Pharmacological treatment
- Reliever therapy – For rapid symptom relief during worsening asthma or exacerbations. May also be used short-term to prevent exercise-induced bronchoconstriction (EIB).
- Maintenance therapy – to reduce airway inflammation, control symptoms & reduce risk of exacerbations and associated decline in lung function.
- Maintenance + Reliever Therapy (MART) → Single inhaler strategy using ICS–formoterol for both daily control and as-needed relief.
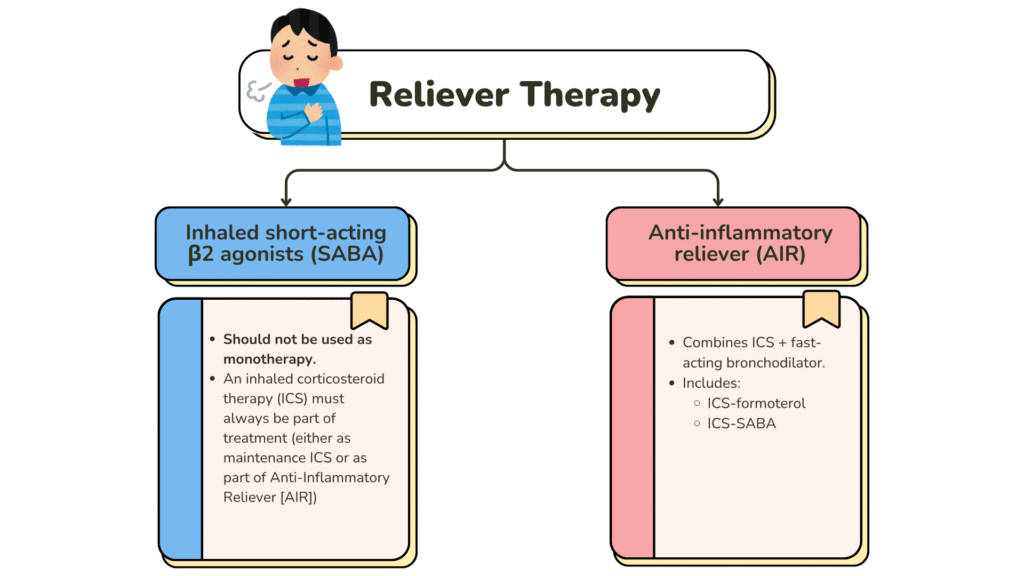
❌Regular SABA monotherapy is not recommended as it is associated with:
- Reduced bronchodilator response
- Increased airway hyperresponsiveness
Oral SABA is not recommended due to higher risk of side effects.
Maintenance therapy (‘Controller’)
Target both symptom control & prevention of future exacerbation risk.
Intended to be used on a regular basis.
Includes:
- Inhaled-corticosteroids (ICS)
– Dosage can be categorized into low-, medium-, or high dose.
– Different ICS within the same category may differ in potency.
– Switching of ICS within a category may change the asthma control.
– Therefore, patients need to be monitored & ICS dose adjusted accordingly.
– High doses of ICS are not recommended for long-term use due to risk of systemic side effects, including easy bruising, increased risk of osteoporosis & fragility fracture, cataracts, glaucoma & adrenal suppression (This is reflected in the GINA 2025 guidelines).
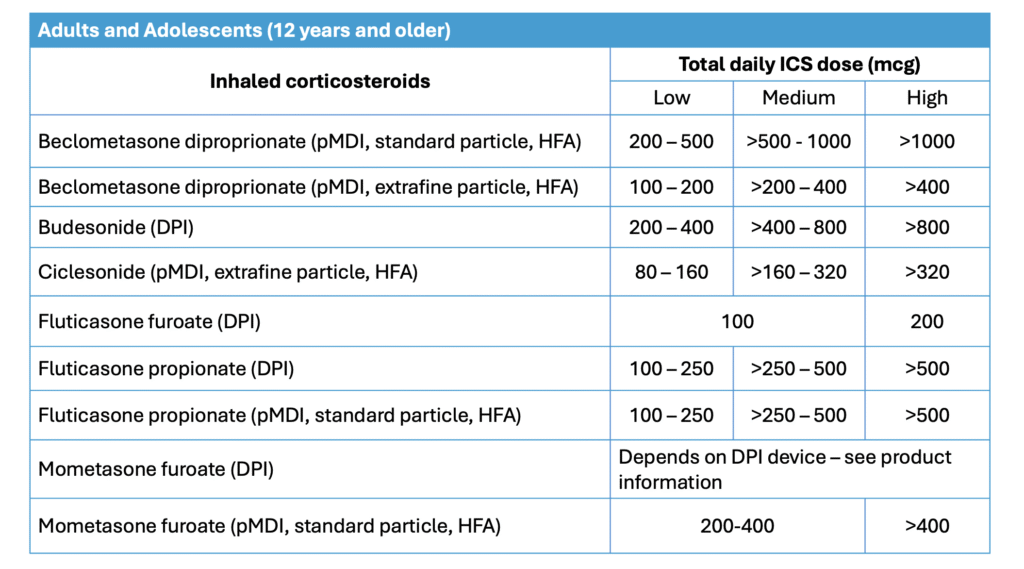
- ICS-LABA combinations
- Others: LTRA, biologic therapy
Maintenance + Reliever Therapy (MART)
Involve the use of a single inhaler containing ICS-formoterol (e.g. budesonide-formoterol or beclomethasone dipropionate-formoterol) as both maintenance & PRN for symptomatic relief.
Others
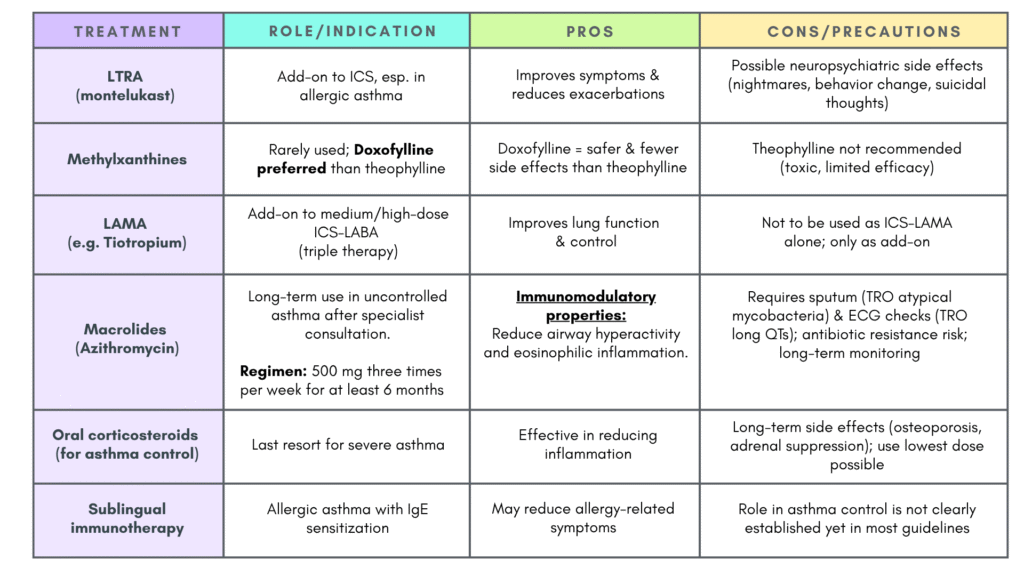
Non-recommended bronchodilators
- Oral bronchodilators e.g. salbutamol tablets/syrups, oral theophylline
- Anticholinergic agents in the absence of ICS
- Formoterol without ICS
