Hyperthyroidism
Terminology
Thyrotoxicosis
High thyroid hormone action in tissues secondary to elevated thyroid hormone levels (can be thought of as symptomatic hyperthyroidism)
Hyperthyroidism
Subset of thyrotoxicosis.
Due to inappropriately high thyroid hormone synthesis & secretion by the thyroid gland.
4 main causes of thyrotoxicosis
Activation of thyroid hormone synthesis & secretion which leads to autonomous release of excessive thyroid hormone.
Examples:
- Graves’ disease
- Toxic adenoma
- Toxic multinodular goitre
- Marine-Lenhart syndrome: Graves + toxic adenoma
Not all hot nodules are actually benign. Some may be malignant & further workout is necessary. Thyroid papillary carcinoma can cause hyperthyroidism
Passively released of preformed hormones from thyroid stores in excessive amounts
Examples:
- Autoimmune: Hashimoto thyroiditis
- Infective: Subacute thyroiditis (viral), MTB, cellulitis of skin anterior to the neck
- Physical insult: rapidly growing thyroid anaplastic carcinoma or primary thyroid lymphoma
Exposure to extrathyroidal sources of thyroid hormone either endogenous or exogenous
Endogenous:
- Struma ovarii (teratoma of the ovaries composed mainly of thyroid tissue)
- Metastatic thyroid carcinoma secreting thyroid hormones.
Exogenous: OTC supplements, cooked animals’ thyroid gland
The thyroid gland is excessively stimulated by trophic factors e.g. thyrotropin-stimulating hormone (TSH) & other factors.
Examples:
- TSHoma (pituitary gland thyrotrophs adenoma)
- Gestational trophoblastic disease –> B-HCG (similar to TSH)
- Jod-Basedov thyrotoxicosis: contrast iodine-induced subclinical hyperthyroidism
- Amiodarone-induced
- L-asparaginase chemotherapy can lead to transient thyrotoxicosis
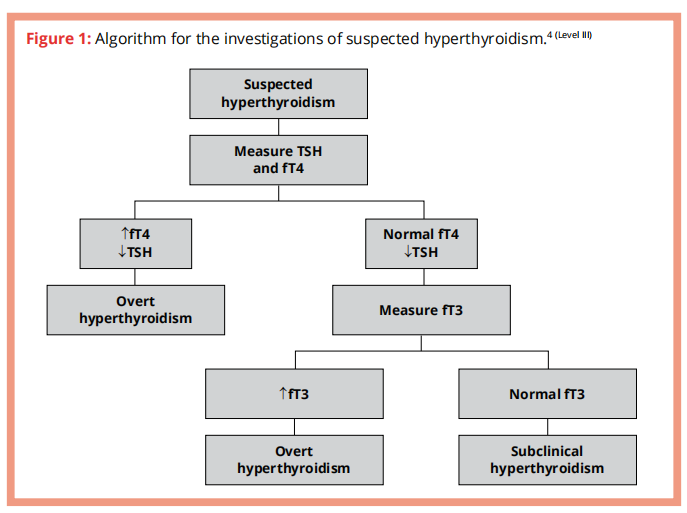
Approach to diagnosis of suspected hyperthyroidism
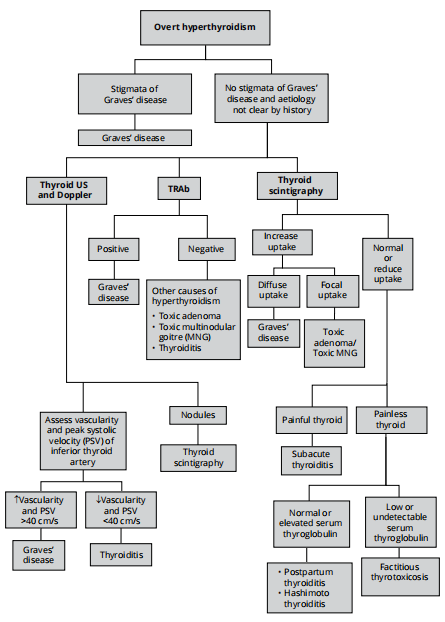
Approach to diagnostic testing of overt hyperthyroidism
Treatment
Graves’ disease
Symptomatic treatment
- Options: propranolol, atenolol, metoprolol, or other beta-blockers.
– CCB, both verapamil & diltiazem may be used to control HR in those who do not tolerate B-blockers. - B-blockers is recommended in all symptomatic patients, especially elderly, those whose resting HR > 90 bpm or having coexistent cardiovascular disease.
Initial treatment options for Graves’ hyperthyroidism
- 131 I therapy (radioactive iodine)
- Antithyroid drugs (ATD)
- Thyroidectomy
Radioactive iodine ☢️
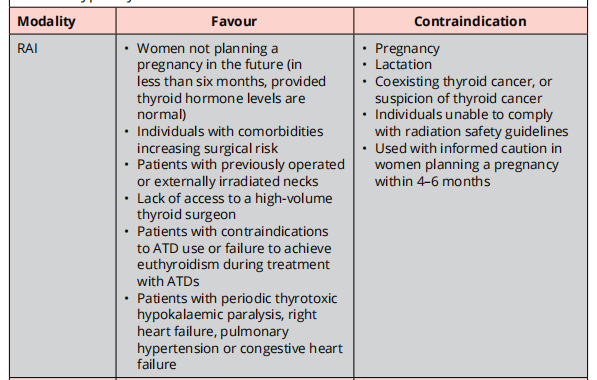
The goal of RAI therapy is to render the patient hypothyroid.
RAI can induce a short-term increase of thyroid hormone levels.
Pretreatment with ATDs prior to RAI should be considered for those who are at increased risk for complications d/t hyperthyroidism. ATDs should be discontinued for 2 – 3 days prior to RAI.
In those who are at increased risk for complications due to worsening of hyperthyroidism, resuming ATDs 3 – 7 days after RAI administration should be considered.
- Those at increased risk of complications include: elderly, those with U/L CVD or severe hyperthyroidism.
If possible, iodinated radiocontrast should be avoided at least 4 – 6 weeks prior to RAI.
Antithyroid drugs 💊
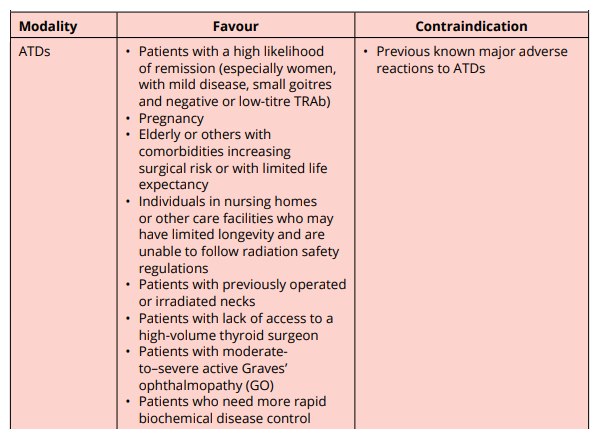
ATDs include carbimazole, methimazole (MMI) and propylthiouracil (PTU)
Goal is to render patient euthyroid
Do not cure Graves.
When they fail to achieve euthyroidism, the usual cause is nonadherence
Carbimazole and MMI (preferred)
- Carbimazole is rapidly converted to MMI in the serum (10 mg of carbimazole is metabolized to ~6mg of MMI)
- Both are effective as OD.
- Start of MMI,
- Initial doses of 10 – 30 mg OD are used to restore euthyroidism, & the dose can then be titrated down to a maintenance (generally 5 – 10 mg OD).
- ADR are more frequent with higher MMI doses.
- When more rapid biochemical control is needed, initial split dose of MMI (e.g. 15 mg or 20 mg BD) may be more effective than a single daily dose because the duration of action of MMI may be less than 24 hours.
- It is important to monitor serum T3 levels initially because some pt normalise their free T4 levels with MMI, but have persistently elevated serum T3, indicating continuing thyrotoxicosis.
Propylthiouracil (PTU)
- MMI has the benefit of OD administration, & reduced risk of major side effects compared to PTU.
- PTU has shorter duration of action.
- Administered BD or TDS
- Starting with 50 – 150 mg TDS
- Maintenance PTU dose of 50 mg BD or TDS
Adverse effects of ATDs
- Common: minor allergic side effects e.g. pruritus or a limited, minor rash (more common with PTU or higher dose MMI 30 mg/day)
- Rare but serious allergic/toxic events: agranulocytosis, vasculitis, or hepatic damage.
A pt is considered to be in remission if they have had a normal serum TSH, free T4, & free T3 for a year after discontinuation of ATD.
Higher initial doses of MMI (60 – 80 mg/day) do not improve remission rates.
– They increase the risk of side effects & are not recommended.
If a patient experiences a relapse at follow up, RAI therapy or surgery can be considered.
If ATD is chosen as the primary therapy, the medication should be continued for ~12 – 18 months, & then discontinued if TSH levels are normal at that time.
– If patient is still hyperthyroid after a course of MMI, can consider treatment with RAI or thyroidectomy.
Thyroidectomy
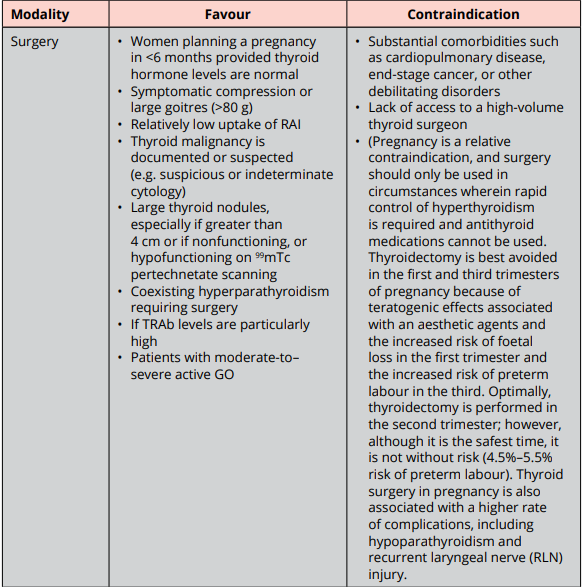
High cure rate for hyperthyroidism of GD.
Options: Near-total or total thyroidectomy.
Most common complications:
- Hypocalcemia d/t hypoparathyroidism (which can be transient or permanent).
- Recurrent or superior laryngeal nerve injury (which can be temporary or permanent).
- Post-op bleeding.
- GA related complications.
If surgery is chose as treatment for GD, pt should be rendered euthyroid prior to the procedure with ATD pretreatment +- b-blockade.

Toxic Multinodular Goitre (TMNG) or Toxic Adenoma (TA)
2 effective & relatively safe options:
- RAI therapy
- Thyroid surgery
Goal of therapy: Rapid & durable elimination of the hyperthyroid state.
Euthyroidism is achieved within days after surgery. However, the risk of hypothyroidism & the requirement for exogenous thyroid hormone therapy is 100% after near-total/total thyroidectomy.
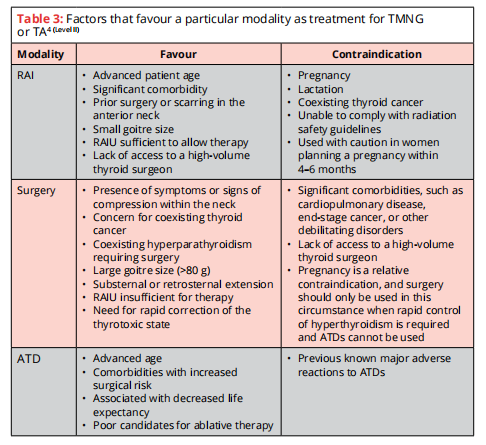
In addition to b-blockade, pretreatment with MMI prior to RAI therapy should be considered in those who are at risk for complications d/t worsening of hyperthyroidism.
If surgery is chosen as treatment, pt with overt hyperthyroidism should be rendered euthyroid prior to the procedure with MMI pretreatment, +- B-blockade.
RAI therapy should be used for retreatment of persistent/recurrent hyperthyroidism following inadequate surgery for TMNG/TA.
Follow up
Post RAI
- Response to RAI can be assessed by monitoring size of the gland, TFT, clinical SSx.
- Normalization of TFT & improvement of clinical Sx within 1 – 2 months.
- Hypothyroidism may occur from 4 weeks onwards, more commonly btw 2 – 6 months.
– Timing of thyroid hormone replacement should be determined by results of TFT, clinical Sx & physical examination.
– Indicated by FT4 below normal range – levothyroxine should be instituted.
– TSH levels may not rise immediately, thus should not be used initially to determine the need for levothyroxine. - Gradual tapering of B-blockers and ATDs.
- Once euthyroid, lifelong annual TFT, or if pt has SSx of hyper/hypothyroidism.
- If hyperthyroidism persist after 6 months, re-treatment with RAI is suggested.
– In some with minimal response 3 months after therapy, additional RAI may be considered. - Refractory hyperthyroidism: consider surgery
Treated with ATD
- FT4 & FT3 at about 2 – 6 weeks to adjust meds accordingly.
– FT3 should be monitored as FT4 may normalize despite persistent elevation of FT3.
– Serum TSH may remain suppressed for several months, thus not recommended to monitor early in the course of therapy. - Once euthyroid, dose of MMI can usually be decreased by 30 – 50% & biochemical testing repeated in 4 – 6 weeks.
– Once on minimal dose of meds, can f/up with clinical & lab evaluation at intervals of 2 – 3 months.
– If pt is on long-term MMI (> 18 months), the interval can be increased to 6 months. - TO TAKE NOTE
– Differential WBC count should be obtained during febrile illness & at the onset of pharyngitis in all pt taking ATD.
– LFT should be assess in pt taking MMI/PTU who experience pruritic rash, jaundice, pale stool or dark urine, joint pain, abdominal pain or bloating, anorexia, nausea, or fatigue.
When to refer
- Hyperthyroidism due to toxic multinodular goitre or toxic adenoma – for RAI/surgical intervention.
- Graves’ disease which failed ATDs or Relapsing Graves’ disease
- Hyperthyroid patient with other comorbidities or who develop comorbidities.
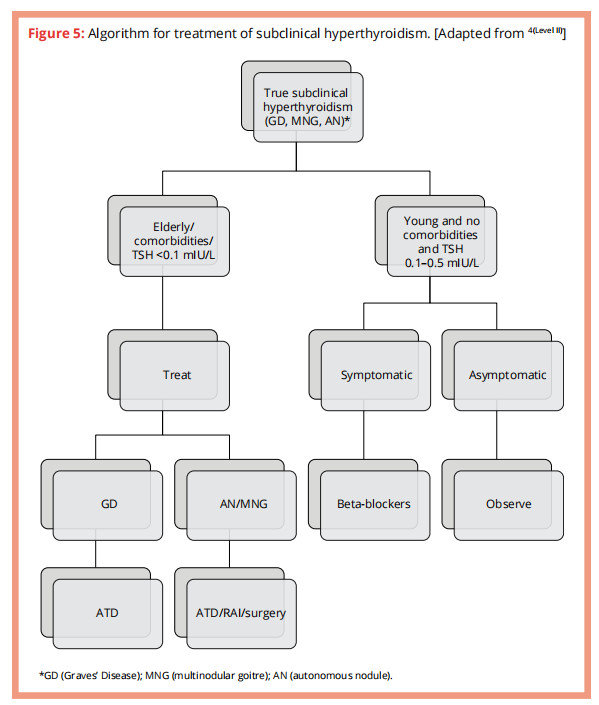
Subclinical hyperthyroidism
Suppressed serum TSH with normal fT3 & fT4 concentrations.
Aetiology similar to that of over hyperthyroidism

Algorithm for approaching subclinical hyperthyroidism
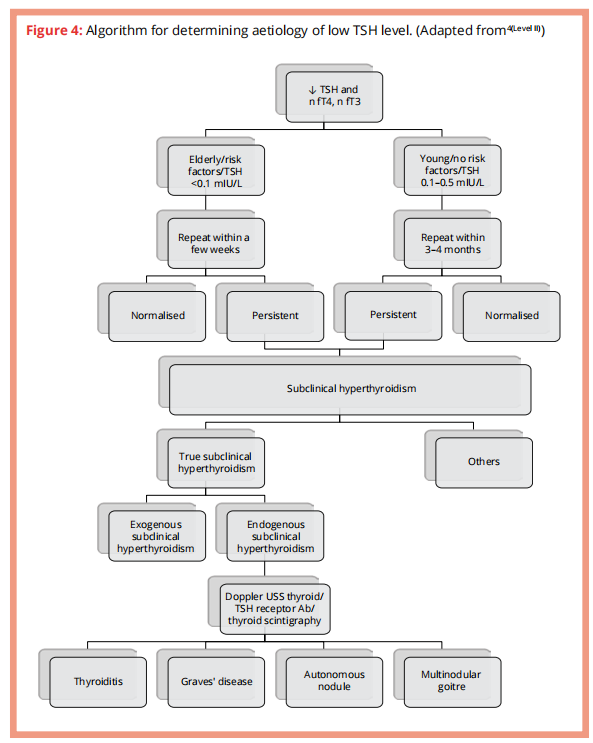
When or who should we treat?
Current evidence suggest that subclinical hyperthyroidism is likely to cause AF, may probably increase the risk of mortality & fractures and possibly cause heart failure & cardiovascular events.
Below are factors to take into consideration when deciding whether to treat or not.
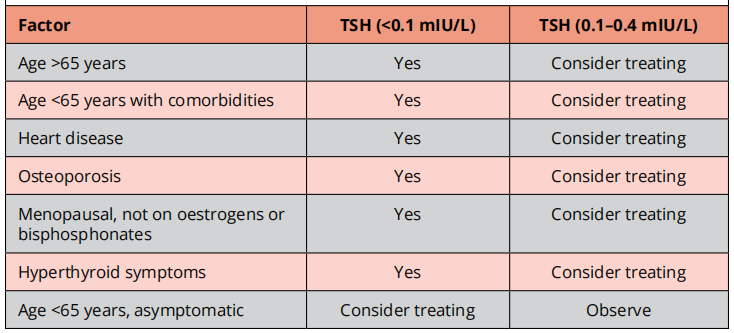
B-blockers should be instituted in patients with subclinical hyperthyroidism.
ATDs should be 1st line and initial treatment for subclinical hyperthyroidism, whatever the aetiology.
Algorithm for treatment