Cervical cancer screening

Cervical Cancer Screening
Natural history of HPV infection & cervical cancer
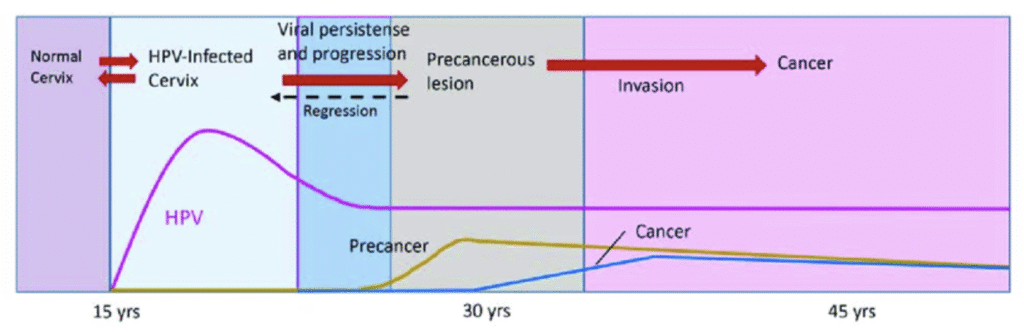
The primary causative agents for cervical cancer is Human Papilloma Virus (HPV), particularly the high-risk type – HPV-16 & HPV-18.
The above graph shows the natural history of HPV infection & cervical cancer:
- The peak prevalence of transient HPV infection can be seen during their teens and 20s after initiation of sexual activity.
- Precancerous condition tend to peak around 10 years later – at the age of 30s (thus the recommendation for starting screening at 30 years of age); whereas the peak prevalence for invasive cancer occurs at around 40 – 50 years of age.
Screening methods for cervical cancer
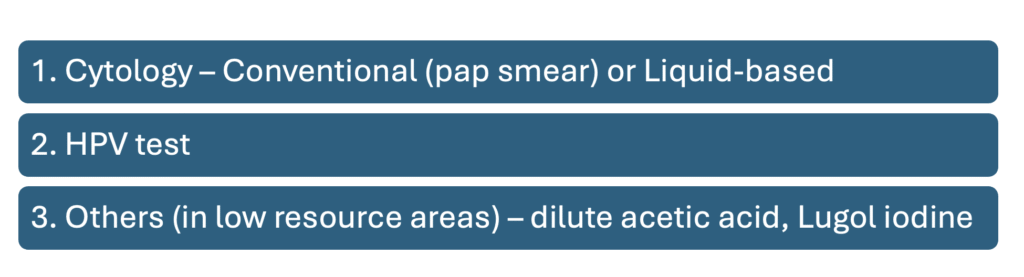
Cytology
Can be either:
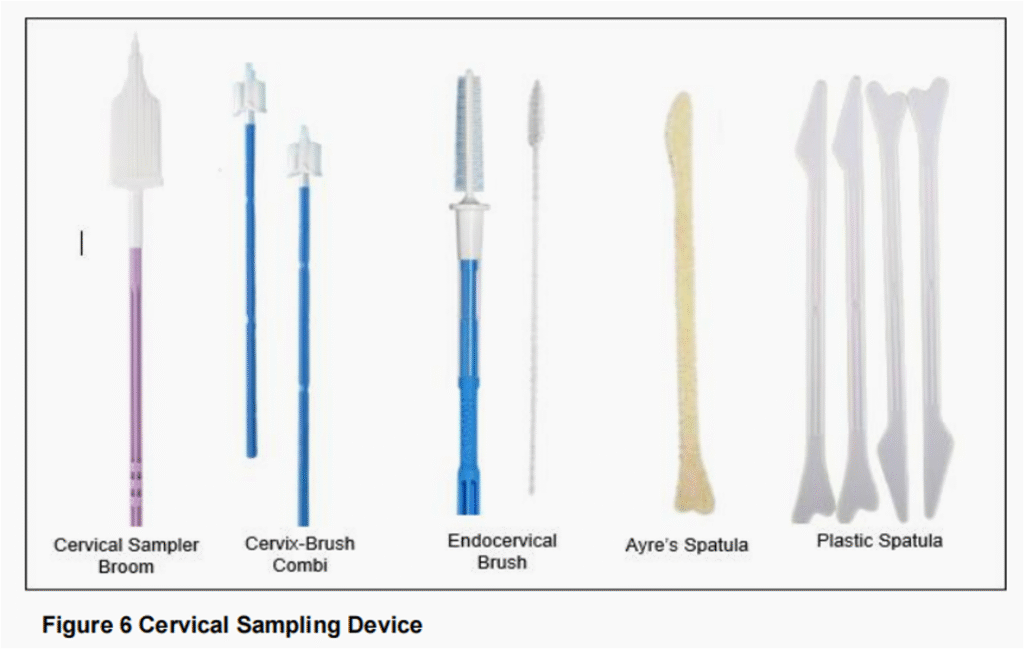
- Conventional cytology (Pap smear) – Sample from cervical scrape is obtained using a cervical brush/broom or spatula, smeared directly onto a glass slide & immediately fixed (within 5 seconds) with 95% alcohol.
- Liquid-based cytology (LBC) – Sample from cervical scrape is obtained using cervical brush/broom & suspended in a vial of preservative for transport to laboratory
Recommended as the screening method for women < 30 years of age.
Screening interval: Initial screening yearly for 2 years, if results normal, then 3-yearly.
LBC is also used as a triage for women screened positive for high-risk HPV (HPV 16/18 & non-16/18) to decided subsequent management
Some common reasons for unsatisfactory cytology:
- Insufficient sample
- Inadequate fixation time (< 30 mins)
- Delay in dipping the slide in the fixative
- Blood-stained smear
- Thick uneven smear
- Excessive discharge/thick inflammatory exudate (on the slide)
- Broken slide beyond repair
- Usage of lubricant before taking smear
- Dirty and contaminated slide
HPV test
HPV test is used as a primary screening in Malaysia for women ≥ 30 years.
Sample collection can be done by healthcare providers or patient themselves (self-sampling)
Screening interval: every 5 years if HPV negative
HPV test may be reported as below:

Report:
HPV 16 – Detected/Not Detected
HPV 18 – Detected/Not Detected
HPV (non 16/18) – Detected/Not Detected
Unsatisfactory
Recommendation for subsequent action
Others (mainly for low resource areas)
Visual inspection with dilute acetic acid (VIA)
- Dilute acetic acid is applied to the cervix, followed by visual examination (without magnification).
- Presence of aceto-white lesion is considered positive & patient will require further evaluation &/or treatment.
Visual inspection with Lugol Iodine (VILI)
- The test is positive if the cervical tissue turns yellow upon Lugol application.
- Inappropriate in postmenopausal women or when the transformation zone is no longer visible.
General advice prior to HPV test/cytology
- Avoid screening during menstruation.
- Avoid sexual intercourse 48 hours prior to procedure
- Do not douche or insert any form of medication or tampons (vaginal creams, foams, films, or spermicides) into the vagina 48 hours before the procedure
- Any cervical lesion seen should be referred to a gynecologist
- Can be performed after 6 weeks postpartum
For procedures on HPV testing and/or cytology, kindly refer to the Guidelines for Cervical Cancer Screening in Malaysia (referenced below) for more detailed walkthrough. Often times, the steps are written on the package insert/user manual that comes along with the instrument.
Here are some points that are important to take note:
- For assisted sampling HPV test by The Healthcare Provider, the sampling is done with the women in the supine position WITHOUT using a speculum.
- The specimen for HPV test should be transported ASAP (not more than 14 days after collection). It should be stored at room temperature (< 30 degrees)
- For cytology, wet the bivalve speculum using sterile water or normal saline (NOT LUBRICANT)
Target age group for screening
Malaysia guideline recommends to start screening at the age of 30.

Note:
- Cervical cancer is rare before age 21, and screening earlier than this age may cause more harm than benefit due to the slow progression of the disease and the high likelihood of regression.
- HPV testing is not recommended as a primary screening for < 30 years
– Reason: Likely to have high rates of positive test due to transient HPV infection that usually resolves spontaneously.
– Potential consequences of positive test: Unnecessary follow-up investigations e.g. colposcopy
Criteria to stop screening ✋
Age ≥ 65:
- 2 negative consecutive HPV tests in the last 10 years.
- Latest test performed within the last 5 years.
Age ≥ 65 with no prior HPV test:
- 2 negative consecutive cytology tests.
- Latest test performed within the last 3 years.
Management according to HPV test results

Repeat unsatisfactory HPV tests within 12 weeks.
Positive HPV results require triage cytology.
Patients with HPV 16/18 but Negative for Intraepithelial Malignancy (NILM) cytology should be referred to a gynecologist for further risk assessment.
Management based on cytology result
If unsatisfactory, repeat within 3 months, if 2nd smear still unsatisfactory, refer for colposcopy
Terminologies:
- NILM: Negative for Intraepithelial Lesion or Malignancy
- ASC-US: Atypical Squamous Cells of Undetermined Significance
- ASC-H: Atypical Squamous Cells, cannot exclude High-grade Squamous Intraepithelial Lesion
- LSIL: Low-grade Squamous Intraepithelial Lesion
- HSIL: High-grade Squamous Intraepithelial Lesion
- AIS: Adenocarcinoma in situ



HSIL/Suspicious for invasion: Refer for colposcopy
SCC, Atypical glandular cells, Adenocarcinoma-in-situ, Adenocarcinoma: Refer gyne/gyne-oncologist.
Endometrial cells seen in women ≥ 45 years of age: Refer for endometrial biopsy
Indications for colposcopy
- Suspicious looking cervix
- Unexplained post-coital bleeding, blood-stained vaginal discharge & postmenopausal bleeding
- Persistent unsatisfactory cytology on 2 occasions, 3 months apart
- Persistent inflammatory cytology on 3 occasions
- Persistent ASC-US on 2 occasions
- ASC-US, positive for high risk HPV
- ASC-H
- Persistent LSIL on 2 occasions, 6 months apart
- LSIL with high risk factors
- HSIL
- SCC
- Atypical Glandular Cells
- Adenocarcinoma
- Positive for high risk HPV DNA with positive cytology
Prevention of cervical cancer
Achieved by HPV vaccination 💉
Available vaccines:
- Bivalent HPV-16/18 vaccine (Cervarix)
- Quadrivalent HPV-6/11/16/18 vaccine (Gardasil)
- Nonavalent HPV-6/11/16/18/31/33/45/52/58 vaccine (Gardasil)
HPV vaccination has been incorporated into our Malaysia National Immunization Programme in 2010
School girls aged 13 years old are given the HPV vaccine, spaced at month 0, 1 and 6 as well as reach completion within the same year.
Though HPV vaccine can be administered to males, routine immunization has not been implemented because male vaccination appears to be less cost-effective according to a US study.
In immunocompromised women
Screening is recommended for all sexually active immunocompromised women.
These include:
- All HIV positive women.
- All women who had undergone solid organ transplant
- All women who are on ≥ 2 immunocompromised medication
Screening modality:
- < 30 years old : Cervical cytology
- ≥ 30 years old : 3-yearly HPV screening
- Those with positive high-risk HPV strains should be sent for colposcopy directly without the need for cytology triage.
Lifetime screening is recommended.
References
- Family Health Development Division Ministry of Health. 2023. Guidelines for Cervical Cancer Screening in Malaysia, Second Edition.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Aug 21;320(7):674-686. doi: 10.1001/jama.2018.10897. PMID: 30140884.
